Animal Experimentation
- Home
- Research and Development
- Research Center Overview
- Animal Experimentation
SR-T100 Preclinical Toxicology and Safety Tests Conducted by Internationally Certified Laboratories Show Excellent Results
| Certified Laboratory | Test Items |
| Development Center for Biotechnology, Toxicology Laboratory | Salmonella Reverse Mutation Test (Ames Test) Human Lymphocyte Chromosomal Aberration Analysis In Vivo Micronucleus Test in Animals Acute Oral Toxicity Test in Mice Acute Oral Toxicity Test in Rats 28-Day Repeated Oral Dose Test in Rats 13-Week Subchronic Oral Dose Toxicity Test in Rats including 4-Week Recovery Group Skin Irritation Test in New Zealand White Rabbits Acute Intravenous Toxicity Test in Rats |
Prostate Enlargement Animal Study Report
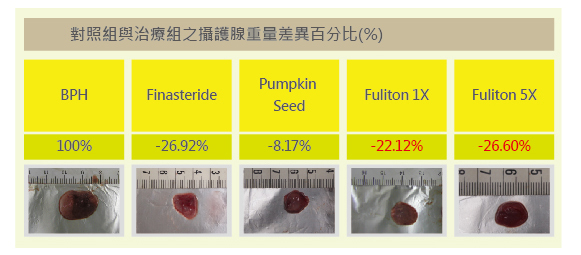
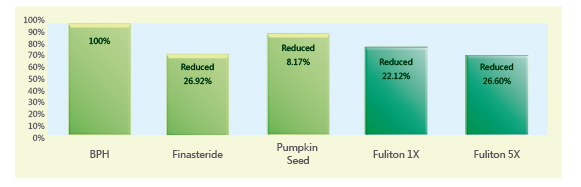
1. Testosterone-Induced Prostate Enlargement Test in Rats
Rats were induced with prostate enlargement through testosterone stimulation. After feeding the test substances to each group, the prostate weights showed the following differences: the group treated with clinical drug Finasteride effectively reduced prostate enlargement (-26.92%) compared to the control group. The pumpkin seed group showed some improvement (-8.17%). The Fuliton group, at the recommended adult dose (1X), achieved almost the same effect as Finasteride (-22.12%). At 5X the recommended dose, Fuliton further reduced prostate weight (-26.60%). Both Fuliton groups (1X and 5X) demonstrated reproducible reductions in prostate enlargement. The results indicate that Fuliton’s efficacy, whether at 1X or 5X dosage, is comparable to Finasteride without its side effects.
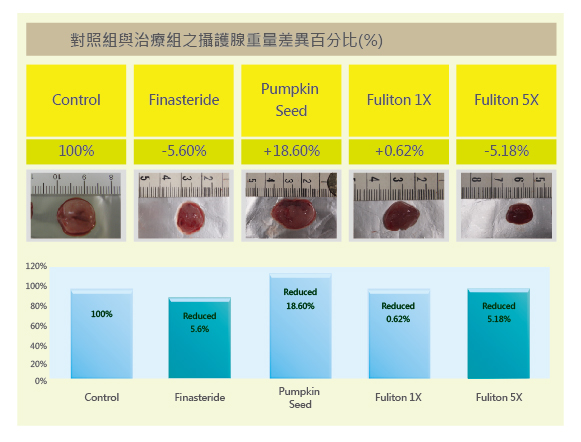
2. Preventive Effect on Age-Related Prostate Enlargement
Rats with testosterone-induced prostate enlargement were treated with the test substances, and prostate weights were compared to naturally aged rats without testosterone stimulation. The Finasteride group effectively reduced enlarged prostate weight to approximately -5.60% relative to natural aging. The pumpkin seed group reduced prostate enlargement slightly, but still had a higher weight compared to natural aging (+18.6%). The Fuliton group, at the recommended adult dose (1X), reduced enlarged prostate weight to a level consistent with natural aging (-0.62%). At 5X the recommended dose, Fuliton further reduced the weight (-5.18%), showing efficacy comparable to the Finasteride group (-5.60%).